Protembis, an Aachen, Germany-based medical-technology company, received €20m in venture-debt financing.
The European Investment Bank (EIB) provided the financing.
The company intends to use the proceeds to:
- complete clinical trials and support marketing of “ProtEmbo” cerebral embolic protection system
- accelerate research, development and market access in Europe, the US and elsewhere
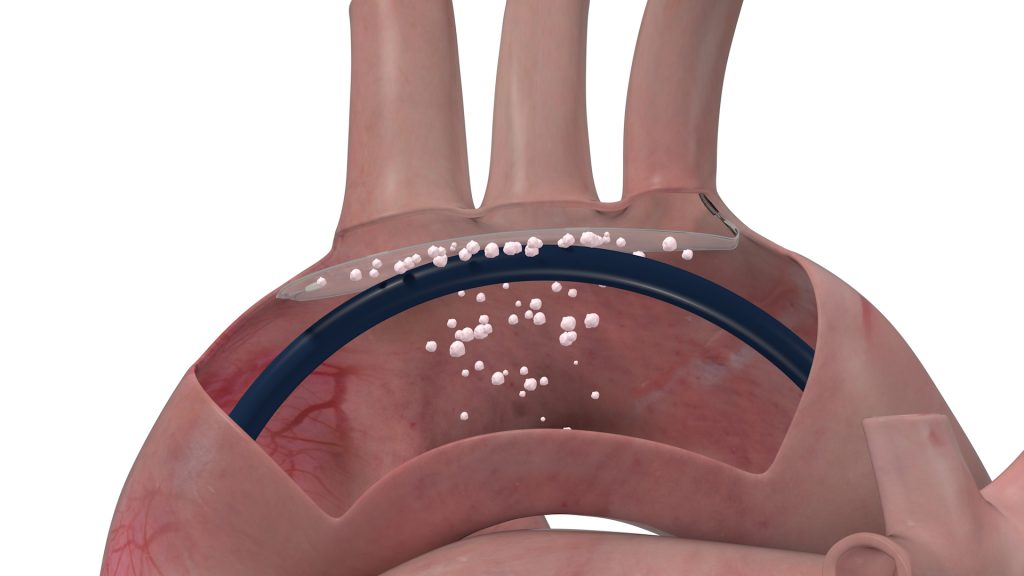
Led by Co-Chief Executive Officers Karl von Mangoldt and Conrad Rasmus, Protembis is advancing a next-generation device for protecting the brains of patients who undergo certain heart treatments. The “ProtEmbo” cerebral embolic protection system is a filter device that deflects embolic material away from arteries to the brain during left-sided heart procedures including transcatheter aortic valve replacement (TAVR), countering risks including stroke and cognitive decline.
In March 2024, Protembis completed a €30m Series B financing round to advance a pivotal FDA approved Investigational Device Exemption study called the ProtEmbo trial. The trial will enrol between 250 and 500 patients undergoing TAVR in Europe and the US.
FinSMEs
02/08/2024